Unravelling human T cell development in health and disease
T cells are a crucial part of the adaptive immune system and their potential to eradicate pathogens and malignant cells is now extensively exploited as an immunotherapeutic tool. T cells are derived from hematopoietic (blood-forming) stem cells and perturbations during their development can result in immunodeficiencies or T cell leukemias. The overall aim of our lab is to uncover the molecular mechanisms that induce and control human T cell development in both normal and malignant settings. By obtaining insights into key mechanisms that drive human T cell development, we not only wish to gather new fundamental knowledge but also aim to utilize these findings towards the design of novel therapeutic applications.
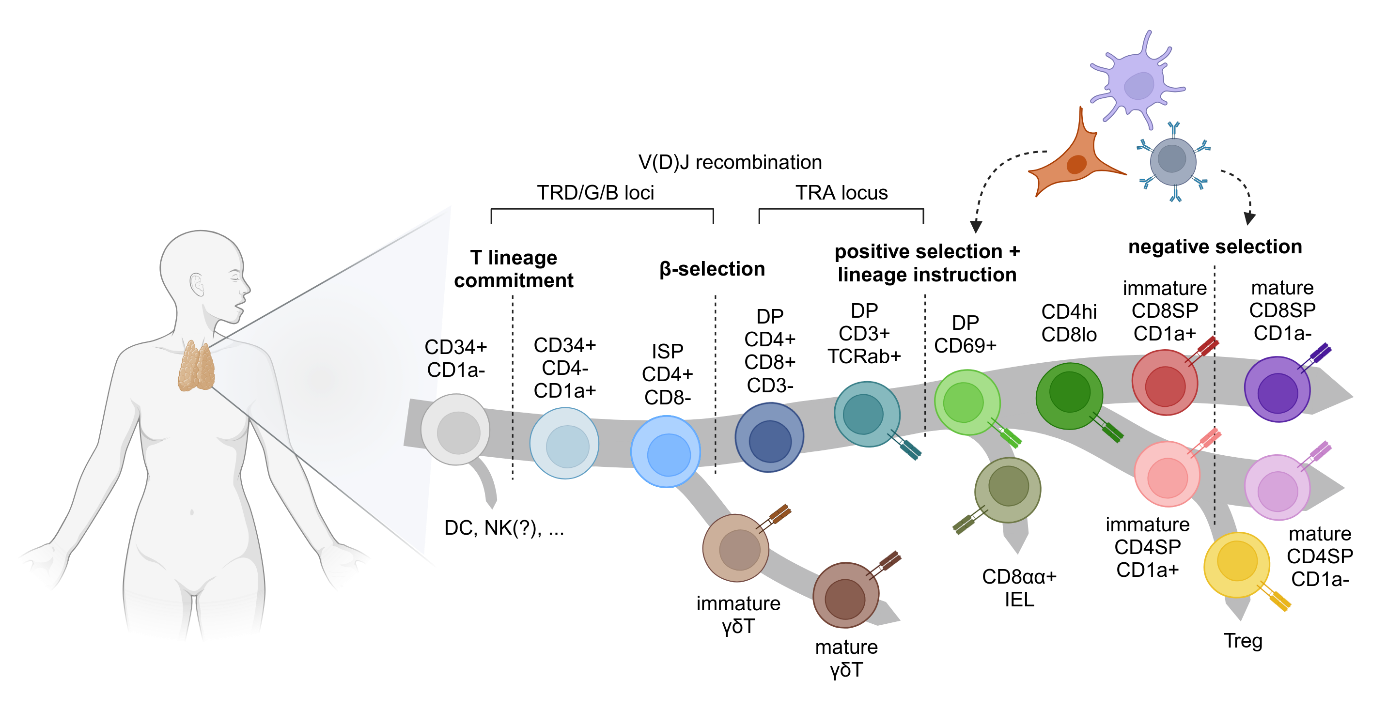 Multipotent hematopoietic stem cells (HSCs), which are maintained in the bone marrow after birth, are capable of generating all the different types of cells in the human blood. To enable their differentiation into T cells, these HSCs must migrate to the micro-environment of the thymus where different stimuli direct these progenitors to generate T lymphocytes. Here, these precursors undergo a step-wise conversion towards mature T cells that is driven by an interplay between the developing T lymphocytes and the thymic stromal cells that also provide the thymic structure. Finally, the fully differentiated T cells migrate to the periphery where they become an essential part of the adaptive immune system.
In a more clinical context, HSCs are transplanted to rebuild the immune system after chemotherapy. The downside of these transplants is the slow and poor reconstitution of T lymphocytes, leading to a prolonged window of immunodeficiency in which patients are highly susceptible to opportunistic infections. By unravelling the molecular network that controls the differentiation of HSCs into T cells, our work might help to improve the generation of T cells following HSC transplantation. This knowledge can also be used to improve the de novo generation of chimeric antigen receptor (CAR) expressing T cells that are increasingly considered as novel cancer treatment. In addition, since many of the key transcription regulators that drive the development of mature T cells are often aberrantly expressed during T cell leukemia, we also aim to reveal how these factors drive oncogenic transformation during T cell differentiation. In this way, by studying the malignant alterations that occur, these fundamental insights might provide novel opportunities for targeted therapy. More details on our research projects can be found in the research section.
The Taghon lab is composed of a young and dynamic international team that uses state-of the art techniques, equipment and know-how to study human T cell development. We are very grateful to the various funding agencies that support our work.
Multipotent hematopoietic stem cells (HSCs), which are maintained in the bone marrow after birth, are capable of generating all the different types of cells in the human blood. To enable their differentiation into T cells, these HSCs must migrate to the micro-environment of the thymus where different stimuli direct these progenitors to generate T lymphocytes. Here, these precursors undergo a step-wise conversion towards mature T cells that is driven by an interplay between the developing T lymphocytes and the thymic stromal cells that also provide the thymic structure. Finally, the fully differentiated T cells migrate to the periphery where they become an essential part of the adaptive immune system.
In a more clinical context, HSCs are transplanted to rebuild the immune system after chemotherapy. The downside of these transplants is the slow and poor reconstitution of T lymphocytes, leading to a prolonged window of immunodeficiency in which patients are highly susceptible to opportunistic infections. By unravelling the molecular network that controls the differentiation of HSCs into T cells, our work might help to improve the generation of T cells following HSC transplantation. This knowledge can also be used to improve the de novo generation of chimeric antigen receptor (CAR) expressing T cells that are increasingly considered as novel cancer treatment. In addition, since many of the key transcription regulators that drive the development of mature T cells are often aberrantly expressed during T cell leukemia, we also aim to reveal how these factors drive oncogenic transformation during T cell differentiation. In this way, by studying the malignant alterations that occur, these fundamental insights might provide novel opportunities for targeted therapy. More details on our research projects can be found in the research section.
The Taghon lab is composed of a young and dynamic international team that uses state-of the art techniques, equipment and know-how to study human T cell development. We are very grateful to the various funding agencies that support our work. 